Primary Focus
Spine Fusion
Non-Alcoholic Steatohepatitis (NASH)
Spine Fusion
Spine fusion is a prevalent procedure performed by orthopedic surgeons and neurosurgeons in order to address spinal deformities and provide stability. Over 400,000 spine fusion surgeries are performed annually in the US. This number is expected to increase significantly over the coming years. Annually, the aggregate hospital cost associated with spine fusion surgeries surpasses $13 billion, the highest of any surgical procedure. Of course, poor clinical outcomes, such as ‘failed fusions’, not only add to this high cost but also cause immense setbacks in patients who often must suffer through repeated surgeries. For example, failed fusions can occur in up to 40% of spine fusion surgeries using autologous bone grafts, even when grafting the patient’s own iliac crest bone, the so called “gold-standard” for these procedures.
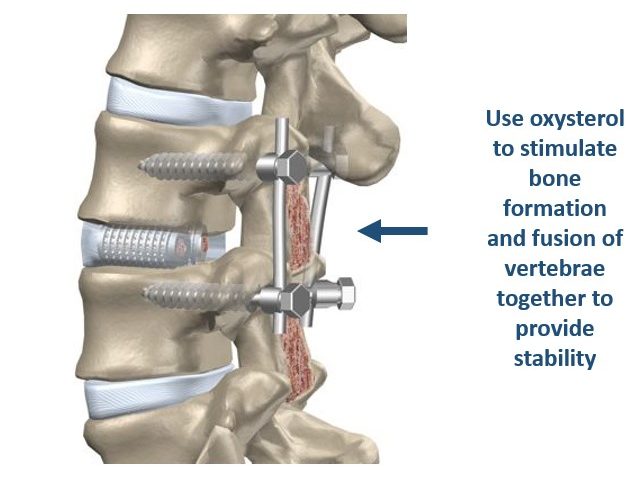
That is why the use of osteoinductive growth factors in bone graft materials, such as recombinant human bone morphogenetic protein 2 (rhBMP2), has become popular, particularly for spine fusion procedures. However, rhBMP2 is produced using expensive recombinant DNA technology and carry their own set of risks, such as uncontrolled or ectopic bone formation, among others. For that reason, we are developing Oxy133, a semi-synthetic osteogenic compound with excellent osteoinductive properties that stimulates bone formation through a different and effective bone forming mechanism than rhBMP2. Oxy133 is an oxysterol-based small molecule, inexpensive and readily produced (compared to recombinant human proteins), shelf stable and easy to use during orthopedic procedures. Oxy133 stimulates bone formation by selectively targeting mesenchymal stem cells as well as other osteoprogenitor cells in the body through allosteric activation of the osteoinductive Hedgehog signaling pathway. Under the right conditions, these cells can transform themselves and help form and repair certain tissues, such as bone, muscle, fat and cartilage. Oxy133 can induce mesenchymal stem cells to differentiate exclusively into bone forming cells (osteoblasts), and prevents them from converting into fat cells (unlike rhBMP2 that stimulates the formation of both bone and fat cells). In humans, the formation of new fat cells in bone often occurs at the expense of bone forming osteoblasts, especially as we age. We believe that Oxy133 could enter the market as a viable alternative to rhBMP2 (Infuse®), a leading product on the market for spine fusion despite its less than favorable safety profile and high cost, and other bone graft materials and lead to improved patient safety and outcomes. We have demonstrated robust bone formation by Oxy133 in animal models of spine fusion, such as in rat and rabbit models as well as the healing of cranial and maxillofacial bone defects. This program is currently about 12 months from entering human clinical trials and the Company has engaged Bruder Consulting and Venture Group (BCVG) that is putting together its pre-IDE and IDE activities toward human clinical trials.
Spine fusion is a prevalent procedure performed by orthopedic surgeons and neurosurgeons in order to address spinal deformities and provide stability. Over 400,000 spine fusion surgeries are performed annually in the US. This number is expected to increase significantly over the coming years. Annually, the aggregate hospital cost associated with spine fusion surgeries surpasses $13 billion, the highest of any surgical procedure. Of course, poor clinical outcomes, such as ‘failed fusions’, not only add to this high cost but also cause immense setbacks in patients who often must suffer through repeated surgeries. For example, failed fusions can occur in up to 40% of spine fusion surgeries using autologous bone grafts, even when grafting the patient’s own iliac crest bone, the so called “gold-standard” for these procedures.
That is why the use of osteoinductive growth factors in bone graft materials, such as recombinant human bone morphogenetic protein 2 (rhBMP2), has become popular, particularly for spine fusion procedures. However, rhBMP2 is produced using expensive recombinant DNA technology and carry their own set of risks, such as uncontrolled or ectopic bone formation, among others. For that reason, we are developing Oxy133, a semi-synthetic osteogenic compound with excellent osteoinductive properties that stimulates bone formation through a different and effective bone forming mechanism than rhBMP2. Oxy133 is an oxysterol-based small molecule, inexpensive and readily produced (compared to recombinant human proteins), shelf stable and easy to use during orthopedic procedures. Oxy133 stimulates bone formation by selectively targeting mesenchymal stem cells as well as other osteoprogenitor cells in the body through allosteric activation of the osteoinductive Hedgehog signaling pathway. Under the right conditions, these cells can transform themselves and help form and repair certain tissues, such as bone, muscle, fat and cartilage. Oxy133 can induce mesenchymal stem cells to differentiate exclusively into bone forming cells (osteoblasts), and prevents them from converting into fat cells (unlike rhBMP2 that stimulates the formation of both bone and fat cells). In humans, the formation of new fat cells in bone often occurs at the expense of bone forming osteoblasts, especially as we age. We believe that Oxy133 could enter the market as a viable alternative to rhBMP2 (Infuse®), a leading product on the market for spine fusion despite its less than favorable safety profile and high cost, and other bone graft materials and lead to improved patient safety and outcomes. We have demonstrated robust bone formation by Oxy133 in animal models of spine fusion, such as in rat and rabbit models as well as the healing of cranial and maxillofacial bone defects. This program is currently about 12 months from entering human clinical trials and the Company has engaged Bruder Consulting and Venture Group (BCVG) that is putting together its pre-IDE and IDE activities toward human clinical trials.
Why Our Work is Important
The global bone grafts and substitutes market was valued at $2.96 billion in 2023 and is expected to reach $4.54 billion by 2030, with a compound annual growth rate (CAGR) of 6.4% for the time-period. Certain areas are projecting higher growth, such as Asia-Pacific, making it the region with the highest projected growth from 2024-2030. Over half of the spine fusion market in 2023 was made up of bone graft substitutes.
Millions of orthopedic procedures are performed annually in the United States. For improved outcomes, many procedures involve the grafting of bone tissue to stimulate bone formation near the site of the procedure for bone strengthening, repair, or stability. Despite the prevalence of these procedures and their clear benefit to patients, poor clinical outcomes leave significant room for improvement in many bone grafting procedures. In fact, there still is a significant unmet medical need for safe, inexpensive and efficacious bone graft materials. Several different types of bone grafting materials are currently available. Using the patient’s own bone as graft material, a so-called autologous bone graft, is associated with significant pain and risk of infection at the site of bone harvesting (e.g., the hip bone). Autologous bone grafts also entail longer recovery times, increased hospitalization cost and risk of other complications. Alternatively, the use of allograft bone grafts, made from cadaver bone, increases the risk of donor-derived infections or rejection of the foreign bone matter by the patient’s immune system. Various synthetic bone graft materials may display decent osteoconductive properties but most often lack strong osteoinductive properties. Oxy133 will be a safer and more effective alternative to autologous and synthetic bone grafts.
Non-Alcoholic Steatohepatitis (NASH)
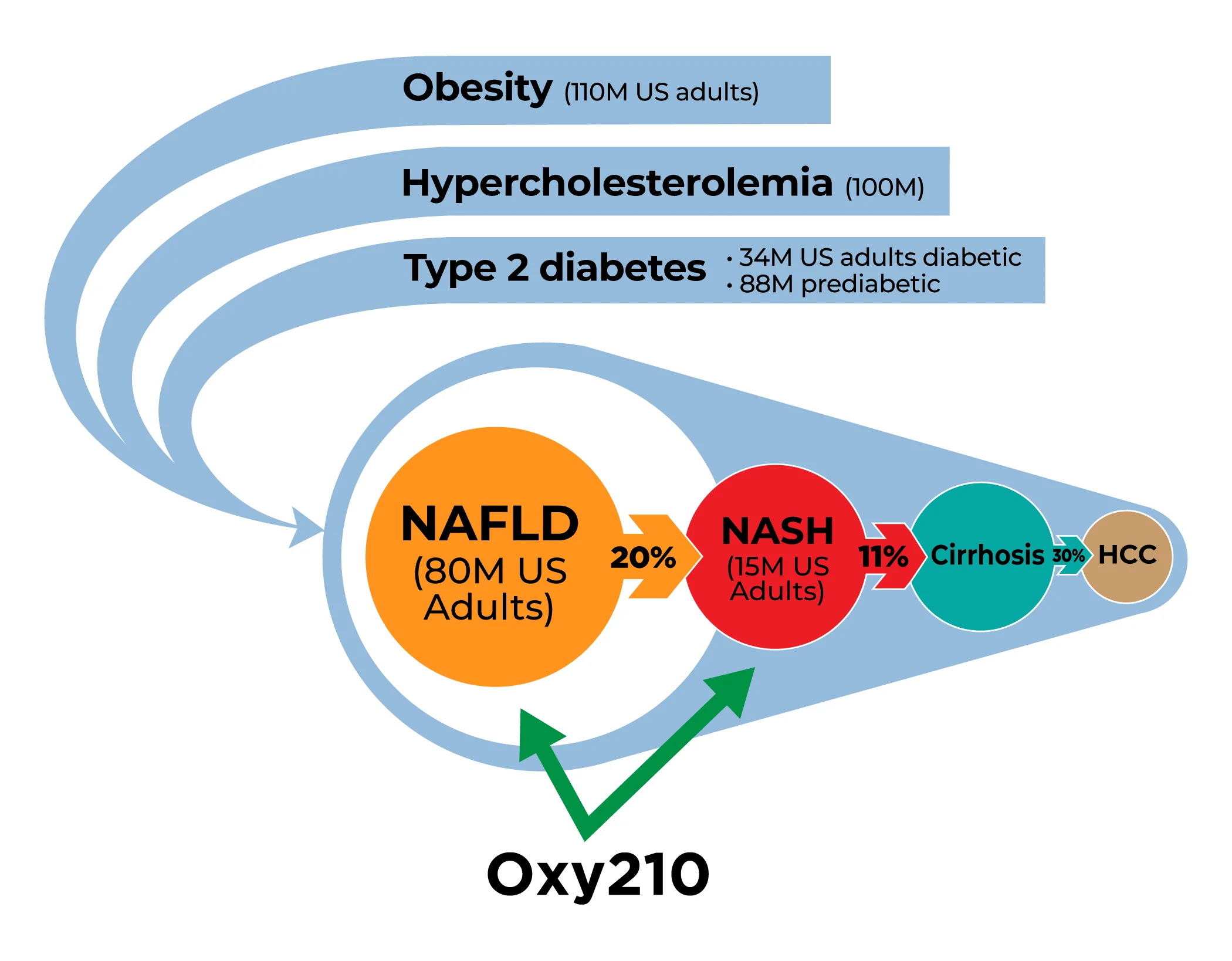
Millions of American adults suffer from obesity, metabolic syndrome, diabetes and cardiovascular disease associated with hyperlipidemia: About 110 million adults in the US are obese; about 88 million, or 34.5% of American adults, are prediabetic; and about 34.2 million Americans suffer from type-2 diabetes. Among this huge patient population, Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD) is very common; in fact, about 80 million, or 30% of the US adult population, suffer from MASLD. Globally, between 20 to 30 percent of the populations in Western developed countries are affected by MASLD. While MASLD can often be benign and reversible with improvements in diet and lifestyle, about 20% of all MASLD patients are at increased risk for developing NASH, a more dangerous inflammatory liver disease that can progress toward liver cirrhosis and liver cancer. Currently, NASH affects an estimated 15 million American adults and this number is expected to rise exponentially in the coming years, driven by the increasing prevalence of obesity, diabetes and metabolic syndrome. The global NASH market size is projected to reach $31 billion by 2027, from $2 billion in 2020, at a CAGR of 46.7% during 2021-2027. In 2017, the direct lifetime costs associated with MASLD/NASH were $222.6 billion; $95.4 billion of this was for patients with NASH.
It is estimated that by 2030, complications resulting from NASH will be the leading cause for liver transplantation procedures in the US. Currently, there are no FDA approved therapies for the treatment of NASH. However, changes in diet and lifestyle and the use of some off-label drugs (e.g., statins) can help support NASH patients, depending on the stage of the disease.
Over the last decades, the development of therapies for NASH has become a high priority of the pharmaceutical industry and a diverse pipeline of innovative drug candidates has been progressing toward the clinic in recent years. However, due to the complex nature of the disease and pipeline attrition, drug failures during late-stage clinical trials have been even more common than in other therapeutic areas, such as cancer, for example. The causes for NASH are multifactorial and incompletely understood, and it seems likely that combination therapies may result in better clinical outcomes than monotherapies, adding additional layers of complication to clinical trial design.
Through our drug discovery platform Oxysterol Therapeutics® technology, we have identified Oxy210, a proprietary oxysterol drug candidate, for targeting NASH. Oxy210 displays a unique combination of anti-inflammatory and anti-fibrotic properties driven in part by inhibiting three major drivers of NASH: 1) transforming growth factor-beta (TGF-β) signaling in hepatic stellate cells (HSC; cells that are responsible for fibrosis in the liver), 2) Hedgehog (Hh) signaling in HSC that causes their activation, and 3) Toll-Like Receptor (TLR) signaling in macrophages that are responsible for inflammation in the liver. In a preclinical humanized mouse model of NASH, Oxy210 significantly inhibited liver inflammation and fibrosis and showed promising disease modifying effects, in addition to favorable drug safety and pharmacokinetic (PK) profiles. Importantly, given that the majority of patients with NASH die of cardiovascular complications, Oxy210 also inhibited atherosclerosis that develops in parallel with NASH through its cholesterol lowering and anti-inflammatory effects.
Why Our Work is Important
We believe that Oxy210 could be developed into a safe and effective treatment for MASLD and NASH patients, as well as individuals with a predisposition to developing these conditions, for example, patients with diabetes and obesity. Oxy210 is orally bioavailable and can be inexpensively produced on scale. Oxy210 is naturally sequestered by the liver after oral administration due to its oxysterol-based properties. Furthermore, Oxy210 may be used as a monotherapy or in combination with other drug candidates that are in the pipeline of other biopharmaceutical companies, for example, farnesoid X receptor (FXR) activators, stearoyl‐CoA desaturase‐1 (SCD-1) modulators, and Glucagon-like peptide-1 (GLP-1) inhibitors. In March 2024 the FDA approved Rezdiffra (resmetirom), an agonist of thyroid hormone receptor-beta (THR-β), indicated for the treatment of non-cirrhotic NASH. In clinical trials, Rezdiffra showed efficacy in less than 30% of patients. By targeting multiple cellular and molecular drivers of NASH, we believe that Oxy210 will distinguish itself from other NASH drug candidates that are specifically designed to affect only a single target.